Indication:
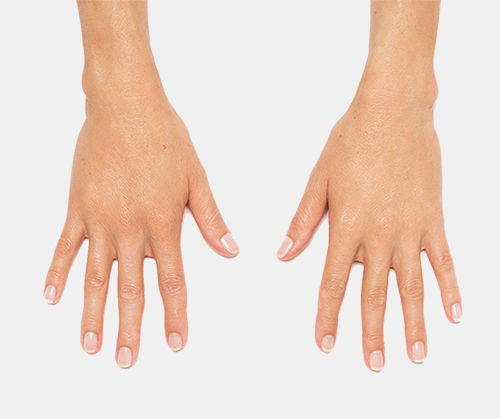
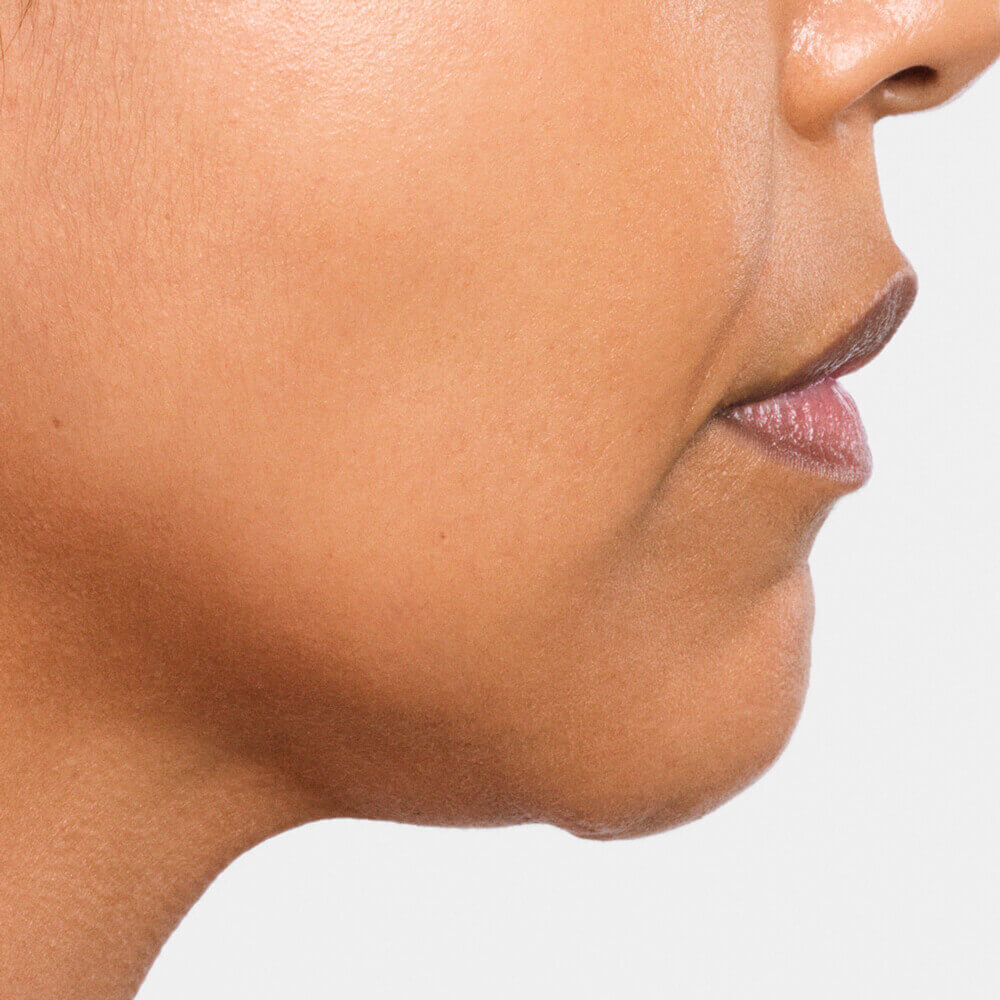
Radiesse© and Radiesse© (+) Injectable Implants are FDA-approved for subdermal implantation for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. RADIESSE® is also indicated for hand augmentation to correct volume loss in the dorsum of the hands. RADIESSE (+) injectable implant is also indicated for deep injection (subdermal and/or supraperiosteal) for soft tissue augmentation to improve moderate to severe loss of jawline contour in adults over the age of 21.
RADIESSE® and RADIESSE® (+) IMPORTANT SAFETY INFORMATION:
Contraindications:
These products are contraindicated for patients with severe allergies manifested by a history of anaphylaxis, or history or presence of multiple severe allergies; patients with known hypersensitivity to any of the components; and patients with bleeding disorders. RADIESSE® (+) is contraindicated in patients with known hypersensitivity to lidocaine or anesthetics of the amide type.
Warnings:
Introduction of product into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft tissue fillers, for example inject the product slowly and apply the least amount of pressure necessary. Rare but serious adverse events associated with the intravascular injection of soft tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage, leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms, including changes in vision, signs of a stroke, blanching of the skin, or unusual pain during or shortly after the procedure. The treating physician should be knowledgeable regarding any pretreatment evaluation and appropriate interventions in the event of intravascular disseminated injection. Prompt intervention by an appropriate medical specialist should be given should these signs or symptoms of intravascular injection occur.
Use of these products in any person with active skin inflammation or infection in or near the treatment should be deferred until the inflammatory or infectious process is controlled.
Do not overcorrect (overfill) a contour deficiency with these products.
Injection into the dorsum of the hand may cause adverse events that last for more than 14 days, and may result in temporary difficulty performing activities (48% of study patients reported this adverse event). RADIESSE® may cause nodules, bumps or lumps in the dorsum of the hand (12% reported this event) and can last up to 1 year.
The safety and effectiveness for use in the lips has not been established. There have been published reports of nodules associated with the use of these products injected into the lips.
Precautions:
In order to minimize the risk of potential complications, this product should only be used by healthcare practitioners who have appropriate training, experience and who are knowledgeable about the anatomy at and around the injection site. Healthcare practitioners should fully familiarize themselves with the product, the product educational materials and the entire package insert.
The safety and effectiveness of RADIESSE® or RADIESSE® (+) in the following situations has not been established:
- Beyond 3 years in the face and 1 year in the hand
- In the periorbital area
- Interactions between RADIESSE® or RADIESSE® (+) and drugs or other substances or implants
- Use during pregnancy, or in breastfeeding women
- In the face in patients under 18 years old
- In the dorsum of the hand in patients under 26 years old and over 79 years old
- In patients with increased susceptibility to keloid formation and hypertrophic scarring
- With concomitant dermal therapies such as epilation, UV irradiation, or laser, mechanical, or chemical peeling procedures
These products contain calcium hydroxylapatite (CaHA) particles that are radiopaque and are clearly visible on CT Scans and may be visible in standard, plain radiography.
As with all transcutaneous procedures, injection of these products carries a risk of infection. Injection in the jawline may temporarily alter jaw function.
To help avoid needle breakage, do not attempt to straighten a bent needle or cannula. Discard it and complete the procedure with a replacement needle.
Patients who are using medications that can prolong bleeding, such as aspirin or warfarin, may experience increased bruising or bleeding at the injection site.
Patients with a history of previous herpetic eruption may experience reactivation of the herpes.
Patients should minimize strenuous activity and exposure of the treated area to extensive sun or heat exposure for approximately 24 hours after treatment or until any initial swelling and redness has resolved.
Adverse Events:
Common adverse events observed in clinical studies of RADIESSE® include bruising, redness, swelling, pain, itching, lumps/bumps at site of injection, difficulty chewing and other local side effects.
Information on adverse events from post-market surveillance of RADIESSE® or RADIESSE® (+) are included in the Instructions for Use (IFU) and Patient Information Guide (PIG) based on an assessment of seriousness and potential causal relationship to RADIESSE® or RADIESSE® (+). Please see the IFU and PIG available at www.radiesse.com for a complete list of these events.
To report a problem with RADIESSE® or RADIESSE® (+), please call MyMerz Solutions at 1-844-469-6379.
For complete Safety Information please refer to the Instructions for Use at www.radiesse.com.
Rx only