More Lift Per Syringe

Radiesse has been used to treat patients in more than 57 countries worldwide. Radiesse was FDA-approved for subdermal implantation for the correction of moderate to severe wrinkles and folds in 2006, and since that time, more than 15 million syringes have been sold worldwide.
Radiesse provides both immediate and long-term rejuvenation:
- immediately smoothing wrinkles and folds
- long-term benefit from the stimulation of new collagen and elastin
Radiesse® 1.5 cc Syringe
Get Results With Less Product
In two separate clinical studies comparing Radiesse to other leading injectable dermal fillers, overall, patients required less Radiesse to achieve full correction in comparison.1,2
- 33% less Radiesse was required for full correction, versus* JUVÉDERM® Injectable Gel1
- 30% less Radiesse was required for full correction, versus* RESTYLANE® Injectable Gel2
Less Radiesse may be required for full correction, giving your practice and your patients more value with each syringe.
*All mention of discontinued products have been removed from this study.
Total Mean Treatment Volume (cc)1
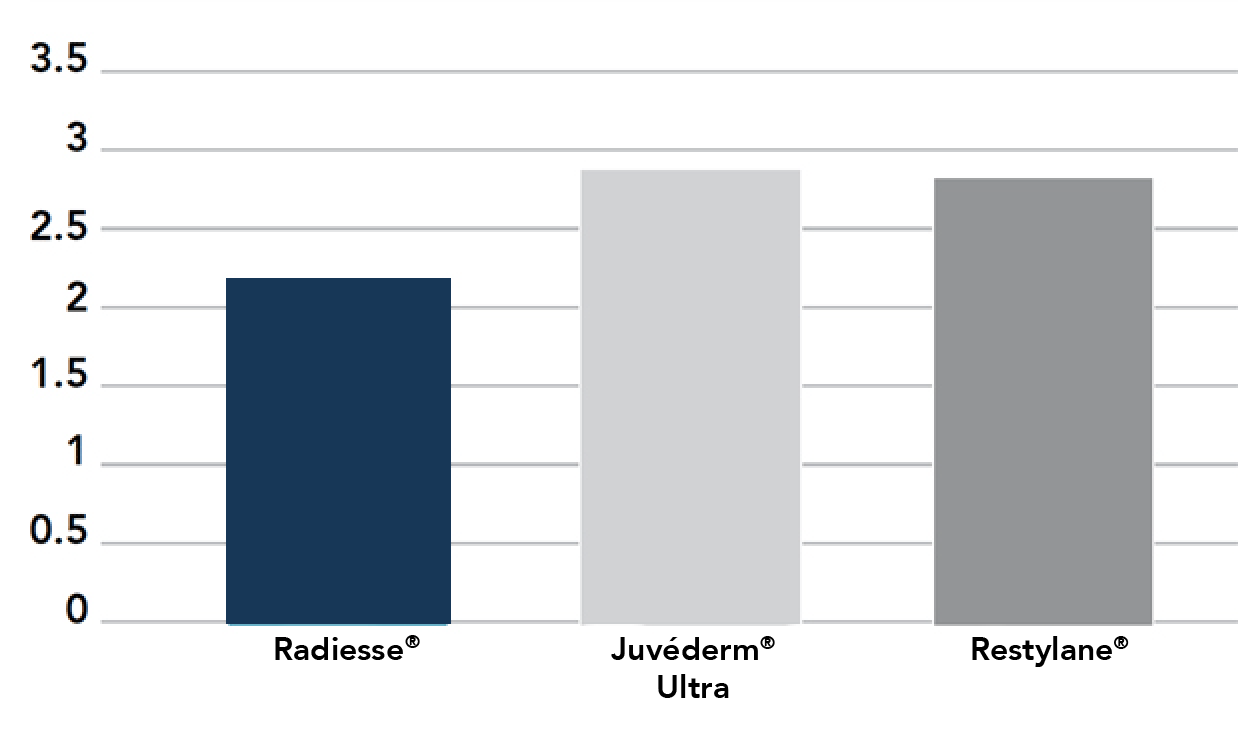
Restylane formally known as Perlane at the time of study.
Trademarks other than Radiesse and Radiesse (+) are the property of their respective owners.
Become a provider
Contact us if you would like more information about becoming a Radiesse provider.
Become A Provider