Learn about Radiesse®
The first and only CaHA injectable treatment in the U.S.
What is CaHA?
Calcium hydroxylapatite, or CaHA, is what makes Radiesse unique as an injectable aesthetic treatment. It’s the first and only CaHA portfolio available that provides both immediate, natural-looking results and long-term improvement—a year or more in many patients.1-8

Immediate contouring plus stimulation of collagen and elastin* production you need1-8
What can Radiesse improve for you?
Radiesse is a one-of-a-kind injectable treatment—the only CaHA that’s FDA approved for use in the lower face and hands.1,2,10

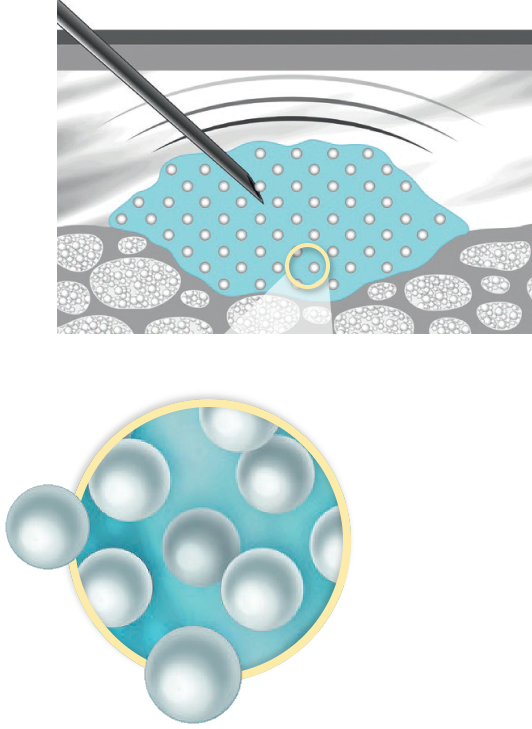
CaHA is the Key
Calcium hydroxylapatite, or CaHA, is what makes Radiesse unique as a dermal filler treatment. RADIESSE® is composed of CaHA suspended in an aqueous gel carrier (ideal for injection).3
Upon injection, the Radiesse CaHA gel matrix provides immediate improvement.3-6
CaHA particles form a “scaffold” that stimulates fibroblasts to produce collagen for up to a year and elastin for up to nine months (after only one injection).5-10
For immediate, long-term rejuvenation:7,11,9
- Immediately smooths wrinkles and folds
- Long-term benefit from the stimulation of new collagen and elastin
Have more questions?
Check out these common questions for more info about Radiesse Injectables and what you might expect from treatment
Ready for a consultation?
Find a provider in your area and schedule a visit to see if a Radiesse treatment is right for you.
